 |
|
| Classification | Baryon |
| Composition | 2 up quarks, 1 down quark |
| Statistics | Fermionic |
| Group | Hadron |
| Interaction | Gravity, Electromagnetic, Weak, Strong |
| Symbol(s) | p, p+ , N+ |
| Antiparticle | Antiproton |
| Theorized | William Prout (1815) |
| Discovered | Ernest Rutherford (1919) |
| Mass | 1.672621777(74)×10−27 kg[1]938.272046(21) MeV/c2[1] |
| Mean lifetime | >2.1×1029 yr (stable) |
| Electric charge | +1 e 1.602176565(35)×10−19 C[1] |
| Charge radius | 0.8775(51) fm[1] |
| Electric dipole moment | <5.4×10−24 e·cm |
| Electric polarizability | 1.20(6)×10−3 fm3 |
| Magnetic moment | 1.410606743(33)×10−26 J·T−1[1]1.521032210(12)×10−3 μB[1] 2.792847356(23) μN[1] |
| Magnetic polarizability | 1.9(5)×10−4 fm3 |
| Spin | 1⁄2 |
| Isospin | 1⁄2 |
| Parity | +1 |
| Condensed | I(JP) = 1⁄2(1⁄2+) |
The proton is a subatomic hadron particle which has the symbol p or p+
and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons.
The proton is also stable by itself. Free protons are emitted directly in some rare types of radioactive decay, and result from the decay of free neutrons from other radioactivity. They soon pick up an electron and become neutral hydrogen, which may then react chemically. Free protons may exist in plasmas or in cosmic rays in vacuum.
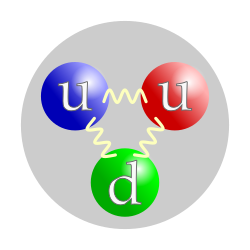
The proton particle is composed of three fundamental particles: two up quarks and one down quark. It is about 1.6–1.7 fm in diameter.[2]
Contents |
Description
Protons are spin-½ fermions and are composed of three quarks,[3] making them baryons (a sub-type of hadrons). The two up quarks and one down quark of the proton are held together by the strong force, mediated by gluons.[2] The proton has an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.[4]
Protons and neutrons are both nucleons, which may be bound by the nuclear force into atomic nuclei. The nucleus of the most common isotope of the hydrogen atom is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium and tritium contain one proton bound to one and two neutrons, respectively. All other types of atoms are composed of two or more protons and various numbers of neutrons. The number of protons in the nucleus determines the chemical properties of the atom and thus which chemical element is represented; it is the number of both neutrons and protons in a nuclide which determine the particular isotope of an element.
Stability
The spontaneous decay of free protons has never been observed, and the proton is therefore considered a stable particle. However, some grand unified theories of particle physics predict that proton decay should take place with lifetimes of the order of 1036 yr, and experimental searches have established lower bounds on the mean lifetime of the proton for various assumed decay products.
Experiments at the Super-Kamiokande detector in Japan gave lower limits for proton mean lifetime of 6.6×1033 yr for decay to an antimuon and a neutral pion, and 8.2×1033 yr for decay to a positron and a neutral pion.[5] Another experiment at the Sudbury Neutrino Observatory in Canada searched for gamma rays resulting from residual nuclei resulting from the decay of a proton from oxygen-16. This experiment was designed to detect decay to any product whatever, and established a lower limit to the proton lifetime of 2.1×1029 yr.[6]
However, protons are known to transform into neutrons through the process of electron capture (also called inverse beta decay). For free protons this process does not occur spontaneously but only when energy is supplied. The equation is:
The process is reversible; neutrons can convert back to protons through beta decay, a common form of radioactive decay. In fact, a free neutron decays this way with a mean lifetime of about 15 minutes.
Quarks and the mass of the proton
In quantum chromodynamics, the modern theory of the nuclear force, most of the mass of the proton and the neutron is explained by special relativity. The mass of the proton is about eighty times greater than the sum of the rest masses of the quarks that make it up, while the gluons have zero rest mass. The extra energy of the quarks and gluons in a region within a proton, as compared to the energy of the quarks and gluons in the QCD vacuum, accounts for over 98% of the mass.
The internal dynamics of the proton are complicated, because they are determined by the quarks exchanging gluons, and interacting with various vacuum condensates. Lattice QCD provides a way of calculating the mass of the proton directly from the theory to any accuracy, in principle. The most recent calculations[7][8] claim that the mass is determined to better than 4% accuracy, arguably accurate to 1% (see Figure S5 in Dürr et al.[8]). These claims are still controversial, because the calculations cannot yet be done with quarks as light as they are in the real world. This means that the predictions are found by a process of extrapolation, which can introduce systematic errors.[9] It is hard to tell whether these errors are controlled properly, because the quantities that are compared to experiment are the masses of the hadrons, which are known in advance.
These recent calculations are performed by massive supercomputers, and, as noted by Boffi and Pasquini: “a detailed description of the nucleon structure is still missing because … long-distance behavior requires a nonperturbative and/or numerical treatment…”[10] More conceptual approaches to the structure of the proton are: the topological soliton approach originally due to Tony Skyrme and the more accurate AdS/QCD approach which extends it to include a string theory of gluons, various QCD inspired models like the bag model and the constituent quark model, which were popular in the 1980s, and the SVZ sum rules which allow for rough approximate mass calculations. These methods don’t have the same accuracy as the more brute force lattice QCD methods, at least not yet.
Charge Radius
The internationally-accepted value of the proton’s charge radius is 0.8768 fm (see orders of magnitude for comparison to other sizes). This value is based on measurements involving a proton and an electron.
However since July 5, 2010 an international research team has been able to make measurements involving a proton and a negatively-charged muon. After a long and careful analysis of those measurements the team concluded that the root-mean-square charge radius of a proton is “0.84184(67) fm, which differs by 5.0 standard deviations from the CODATA value of 0.8768(69) fm.”[11]
The international research team that obtained this result at the Paul-Scherrer-Institut (PSI) in Villigen (Switzerland) includes scientists from the Max Planck Institute of Quantum Optics (MPQ) in Garching, the Ludwig-Maximilians-Universität (LMU) Munich and the Institut für Strahlwerkzeuge (IFWS) of the Universität Stuttgart (both from Germany), and the University of Coimbra, Portugal.[12][13] They are now attempting to explain the discrepancy, and re-examining the results of both previous high-precision measurements and complicated calculations. If no errors are found in the measurements or calculations, it could be necessary to re-examine the world’s most precise and best-tested fundamental theory: quantum electrodynamics.[14]
Proton in chemistry
Atomic number
In chemistry the number of protons in the nucleus of an atom is known as the atomic number, which determines the chemical element to which the atom belongs. For example, the atomic number of chlorine is 17; this means that each chlorine atom has 17 protons and that all atoms with 17 protons are chlorine atoms. The chemical properties of each atom are determined by the number of (negatively charged) electrons, which for neutral atoms is equal to the number of (positive) protons so that the total charge is zero. For example, a neutral chlorine atom has 17 protons and 17 electrons, while a negative Cl− ion has 17 protons and 18 electrons for a total charge of −1.
All atoms of a given element are not necessarily identical, however, as the number of neutrons may vary to form different isotopes, and energy levels may differ forming different nuclear isomers. For example, there are two stable isotopes of chlorine: 35
17Cl with 35 – 17 = 18 neutrons and 37
17Clwith 37 – 17 = 20 neutrons.
Hydrogen ion
In chemistry the term proton refers to the hydrogen ion, H+. Since the atomic number of hydrogen is 1, a hydrogen ion has no electrons and corresponds to a bare nucleus, consisting of a proton (and 0 neutrons for the most abundant isotope protium 1
1H). The proton itself is some 1800 times smaller than a hydrogen atom and so is extremely reactive. The free proton has an extremely short lifetime in chemical systems. It reacts rapidly with any available molecule. In aqueous solution it forms the hydronium ion, which in turn is further solvated by water molecules in clusters such as [H5O2]+ and [H9O4]+.[15]
The transfer of H+ in an acid–base reaction is usually referred to as “proton transfer”. The acid is referred to as a proton donor and the base as a proton acceptor. Similarly, biochemical terms such as proton pump and proton channel refer to the movement of hydrated H+ ions.
The ion produced by removing the electron from a deuterium atom is known as a deuteron. The negatively charged ion H− is known as the hydride ion. D− is known as the deuteride ion. tritium is used for isotopic labelling of organic compounds. Tritium ions are rarely studied in chemistry.
Proton nuclear magnetic resonance (NMR)
Also in chemistry, the term “proton NMR” refers to the observation of hydrogen-1 nuclei in (mostly organic) molecules by nuclear magnetic resonance. This method uses the spin of the proton, which has the value one-half. The name refers to examination of protons as they occur in protium (hydrogen-1 atoms) in compounds, and does not imply that free protons exist in the compound being studied.
History
The concept of a hydrogen-like particle as a constituent of other atoms was developed over a long period. As early as 1815, William Prout proposed that all atoms are composed of hydrogen atoms, based on a simplistic interpretation of early values of atomic weights (see Prout’s hypothesis), which was disproved when more accurate values were measured.
In 1886 Eugen Goldstein discovered canal rays (also known as anode rays) and showed that they were positively charged particles (ions) produced from gases. However, since particles from different gases had different values of charge-to-mass ratio (e/m), they could not be identified with a single particle, unlike the negative electrons discovered by J. J. Thomson.
Following the discovery of the atomic nucleus by Ernest Rutherford in 1911, Antonius van den Broek proposed that the place of each element in the periodic table (its atomic number) is equal to its nuclear charge. This was confirmed experimentally by Henry Moseley in 1913 using X-ray spectra. In 1917 (in experiments reported in 1919) Rutherford proved that the hydrogen nucleus is present in other nuclei, a result usually described as the discovery of the proton.[16] He noticed that when alpha particles were shot into air, and (after experimentation) to a higher degree into pure nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that this hydrogen could only have come from the nitrogen, and therefore nitrogen must contain hydrogen nuclei. One hydrogen nucleus was being knocked off by the impact of the alpha particle, producing oxygen-17 in the process. This was the first reported nuclear reaction, 14N + α → 17O + p. The hydrogen nucleus is therefore present in other nuclei as an elementary particle, which Rutherford named the proton, after the neuter singular of the Greek word for “first”, πρῶτον.
Exposure
The Apollo Lunar Surface Experiments Packages (ALSEP) determined that more than 95% of the particles in the solar wind are electrons and protons, in approximately equal numbers.[17][18]
Because the Solar Wind Spectrometer made continuous measurements, it was possible to measure how the Earth’s magnetic field affects arriving solar wind particles. For about two-thirds of each orbit, the Moon is outside of the Earth’s magnetic field. At these times, a typical proton density was 10 to 20 per cubic centimeter, with most protons having velocities between 400 and 650 kilometers per second. For about five days of each month, the Moon is inside the Earth’s geomagnetic tail, and typically no solar wind particles were detectable. For the remainder of each lunar orbit, the Moon is in a transitional region known as the magnetosheath, where the Earth’s magnetic field affects the solar wind but does not completely exclude it. In this region, the particle flux is reduced, with typical proton velocities of 250 to 450 kilometers per second. During the lunar night, the spectrometer was shielded from the solar wind by the Moon and no solar wind particles were measured.[17]
Research has been performed on the dose-rate effects of protons, as typically found in space travel, on human health.[18][19] More specifically, there are hopes to identify what specific chromosomes are damaged, and to define the damage, during cancer development from proton exposure.[18] Another study looks into determining “the effects of exposure to proton irradiation on neurochemical and behavioral endpoints, including dopaminergic functioning, amphetamine-induced conditioned taste aversion learning, and spatial learning and memory as measured by the Morris water maze.”[19] Electrical charging of a spacecraft due to interplanetary proton bombardment has also been proposed for study.[20] There are many more studies which pertain to space travel, including galactic cosmic rays and their possible health effects, and solar proton event exposure.
The American Biostack and Soviet Biorack space travel experiments have demonstrated the severity of molecular damage induced by heavy ions on micro organisms including Artemia cysts.[21]
Antiproton
CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles and, therefore, is open to stringent tests. For example, the charges of the proton and antiproton must sum to exactly zero. This equality has been tested to one part in 108. The equality of their masses has also been tested to better than one part in 108. By holding antiprotons in a Penning trap, the equality of the charge to mass ratio of the proton and the antiproton has been tested to one part in 6×109.[22] The magnetic moment of the antiproton has been measured with error of 8×10−3 nuclear Bohr magnetons, and is found to be equal and opposite to that of the proton.
See also
References
- ^ a b c d e f g h P.J. Mohr, B.N. Taylor, and D.B. Newell (2011), “The 2010 CODATA Recommended Values of the Fundamental Physical Constants” (Web Version 6.0). This database was developed by J. Baker, M. Douma, and S. Kotochigova. Available: http://physics.nist.gov/constants [Thursday, 02-Jun-2011 21:00:12 EDT]. National Institute of Standards and Technology, Gaithersburg, MD 20899.
- ^ a b W.N. Cottingham, D.A. Greenwood (1986). An Introduction to Nuclear Physics. Cambridge University Press. p. 19.
- ^ R.K. Adair (1989). The Great Design: Particles, Fields, and Creation. Oxford University Press. p. 214.
- ^ J.-L. Basdevant, J. Rich, M. Spiro (2005). Fundamentals in Nuclear Physics. Springer. p. 155. ISBN 0-387-01672-4. http://books.google.com/?id=OFx7P9mgC9oC&pg=PA375&dq=helium+%22nuclear+structure%22.
- ^ H. Nishino et al. (Kamiokande collaboration) (2009). “Search for Proton Decay via p → e+
π0
and p → μ+
π0
in a Large Water Cherenkov Detector”. Physical Review Letters 102: 141801. Bibcode 2009PhRvL.102n1801N. doi:10.1103/PhysRevLett.102.141801. - ^ S.N. Ahmed et al. (SNO Collaboration) (2004). “Constraints on nucleon decay via invisible modes from the Sudbury Neutrino Observatory”. Physical Review Letters 92: 102004. arXiv:hep-ex/0310030. Bibcode 2004PhRvL..92j2004A. doi:10.1103/PhysRevLett.92.102004. PMID 15089201.
- ^ See this news report and links
- ^ a b S. Dürr, Z. Fodor, J. Frison, C. Hoelbling, R. Hoffmann, S. D. Katz, S. Krieg, T. Kurth, L. Lellouch, T. Lippert, K. K. Szabo, and G. Vulvert (21 November 2008). “Ab Initio Determination of Light Hadron Masses”. Science 322 (5905): 1224. Bibcode 2008Sci…322.1224D. doi:10.1126/science.1163233. PMID 19023076. http://www.sciencemag.org/cgi/data/322/5905/1224/DC1/1.
- ^ C. F. Perdrisat, V. Punjabi, M. Vanderhaeghen (2007). “Nucleon Electromagnetic Form Factors”. Prog Part Nucl Phys 59: 694–764. arXiv:hep-ph/0612014. Bibcode 2007PrPNP..59..694P. doi:10.1016/j.ppnp.2007.05.001.
- ^ Sigfrido Boffi & Barbara Pasquini (2007). “Generalized parton distributions and the structure of the nucleon”. Riv Nuovo Cim 30. arXiv:0711.2625. Bibcode 2007NCimR..30..387B. doi:10.1393/ncr/i2007-10025-7.
- ^ Randolf Pohl, Aldo Antognini, François Nez, Fernando D. Amaro, François Biraben, João M. R. Cardoso, Daniel S. Covita, Andreas Dax, Satish Dhawan, Luis M. P. Fernandes, Adolf Giesen, Thomas Graf, Theodor W. Hänsch, Paul Indelicato, Lucile Julien, Cheng-Yang Kao, Paul Knowles, Eric-Olivier Le Bigot, Yi-Wei Liu, José A. M. Lopes, Livia Ludhova, Cristina M. B. Monteiro, Françoise Mulhauser, Tobias Nebel, Paul Rabinowitz, et al. (8 July 2010). “The size of the proton“. Nature 466 (7303): 213–216. Bibcode 2010Natur.466..213P. doi:10.1038/nature09250. PMID 20613837. http://www.nature.com/nature/journal/v466/n7303/abs/nature09250.html. Retrieved 2010-07-09.
- ^ New proton measurements may throw physics a curve
- ^ “The Proton Just Got Smaller”. Photonics.Com. July 12, 2010. http://www.photonics.com/Article.aspx?AID=42905. Retrieved 2010-07-19.
- ^ Researchers Observes Unexpectedly Small Proton Radius in a Precision Experiment
- ^ Headrick, J.M.; Diken, E.G.; Walters, R. S.; Hammer, N. I.; Christie, R.A. ; Cui, J.; Myshakin, E.M.; Duncan, M.A.; Johnson, M.A.; Jordan, K.D. (2005). “Spectral Signatures of Hydrated Proton Vibrations in Water Clusters”. Science 308 (5729): 1765–69. Bibcode 2005Sci…308.1765H. doi:10.1126/science.1113094. PMID 15961665.
- ^ R.H. Petrucci, W.S. Harwood, and F.G. Herring (2002). General Chemistry (8th ed.). p. 41.
- ^ a b “Apollo 11 Mission”. Lunar and Planetary Institute. 2009. http://www.lpi.usra.edu/lunar/missions/apollo/apollo_11/experiments/swc/. Retrieved 2009-06-12.
- ^ a b c “Space Travel and Cancer Linked? Stony Brook Researcher Secures NASA Grant to Study Effects of Space Radiation”. Brookhaven National Laboratory. 12 December 2007. http://www.bnl.gov/bnlweb/pubaf/pr/PR_display.asp?prID=07-X17. Retrieved 2009-06-12.
- ^ a b B. Shukitt-Hale, A. Szprengiel, J. Pluhar, B.M. Rabin, and J.A. Joseph. “The effects of proton exposure on neurochemistry and behavior”. Elsevier/COSPAR. http://biblioteca.universia.net/ficha.do?id=43176300. Retrieved 2009-06-12.
- ^ N.W. Green and A.R. Frederickson. “A Study of Spacecraft Charging due to Exposure to Interplanetary Protons”. Jet Propulsion Laboratory. http://trs-new.jpl.nasa.gov/dspace/bitstream/2014/39501/1/05-0657.pdf. Retrieved 2009-06-12.
- ^ H. Planel (2004). Space and life: an introduction to space biology and medicine. CRC Press. pp. 135–138. ISBN 0415317592. http://books.google.com/?id=rnUFZ24RUdYC&pg=PA132&lpg=PA132&dq=space+colonization+proton+exposure&q=.
- ^ G. Gabrielse (2006). “Antiproton mass measurements”. International Journal of Mass Spectrometry 251 (2–3): 273–280. Bibcode 2006IJMSp.251..273G. doi:10.1016/j.ijms.2006.02.013.
External links
| Wikimedia Commons has media related to: Proton |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This information originally retrieved from http://en.wikipedia.org/wiki/Proton
on Wednesday 3rd August 2011 1:29 pm EDT
Now edited and maintained by ManufacturingET.org
