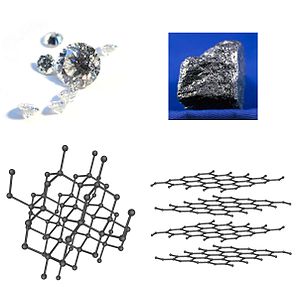
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements. Allotropes are different structural modifications of an element;[1] the atoms of the element are bonded together in a different manner.
Take carbon for example: 4 common allotropes of carbon are diamond (where the carbon atoms are bonded together in a tetrahedral lattice arrangement), graphite (where the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (where the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations).
The term allotropy is used for elements only, not for compounds. The more general term, used for any crystalline material, is polymorphism. Allotropy refers only to different forms of an element within the same phase (i.e. different solid, liquid or gas forms); the changes of state between solid, liquid and gas in themselves are not considered allotropy.
For some elements, allotropes have different molecular formulae which can persist in different phases – for example, two allotropes of oxygen (dioxygen, O2 and ozone, O3), can both exist in the solid, liquid and gaseous states. Conversely, some elements do not maintain distinct allotropes in different phases – for example phosphorus has numerous solid allotropes, which all revert to the same P4 form when melted to the liquid state.
Contents |
History
The concept of allotropy was originally proposed in 1841 by the Swedish scientist Baron Jöns Jakob Berzelius (1779–1848) who offered no explanation.[2] The term is derived from the Greek άλλοτροπἱα (allotropia; variability, changeableness).[3] After the acceptance of Avogadro’s hypothesis in 1860 it was understood that elements could exist as polyatomic molecules, and the two allotropes of oxygen were recognized as O2 and O3. In the early 20th century it was recognized that other cases such as carbon were due to differences in crystal structure.
By 1912, Ostwald noted that the allotropy of elements is just a special case of the phenomenon of polymorphism known for compounds, and proposed that the terms allotrope and allotropy be abandoned and replaced by polymorph and polymorphism. Although many other chemists have repeated this advice, IUPAC and most chemistry texts still favour the usage of allotrope and allotropy for elements only.
Differences in properties of an element’s allotropes
Allotropes are different structural forms of the same element and can exhibit quite different physical properties and chemical behaviours. The change between allotropic forms is triggered by the same forces that affect other structures, i.e. pressure, light, and temperature. Therefore the stability of the particular allotropes depends on particular conditions. For instance, iron changes from a body-centered cubic structure (ferrite) to a face-centered cubic structure (austenite) above 906 °C, and tin undergoes a transformation known as tin pest from a metallic phase to a semiconductor phase below 13.2 °C. As an example of different chemical behaviour, ozone (O3) is a much stronger oxidizing agent than dioxygen (O2).
List of allotropes
Typically, elements capable of variable coordination number and/or oxidation states tend to exhibit greater numbers of allotropic forms. Another contributing factor is the ability of an element to catenate. Allotropes are typically more noticeable in non-metals (excluding the halogens and the noble gases) and metalloids. Nevertheless, metals tend to have many allotropes.
Examples of allotropes include:
Non-metals and metalloids
| Element | Allotropes |
|---|---|
| Carbon |
|
| Phosphorus: |
|
| Oxygen: |
|
| Sulfur: |
|
| Selenium: |
|
| Boron |
|
| Germanium |
|
| Silicon |
|
| Arsenic: |
|
| Antimony: |
|
Metals
Among the naturally occurring metallic elements (up to U, without Tc and Pm), 28 are allotropic at ambient pressure: Li, Be, Na, Ca, Sr, Ti, Mn, Fe, Co, Sr, Y, Zr, Sn, La, Ce, Pr, Nd, (Pm), Sm, Gd, Tb, Dy, Yb, Hf, Tl, Po, Th, Pa, U. Considering only the technologically-relevant metals,[clarification needed] six metals are allotropic: Ti at 882˚C, Fe at 912˚C and 1394˚C, Co at 422˚C, Zr at 863˚C, Sn at 13˚C and U at 668˚C and 776˚C.
- grey tin (alpha-tin)
- white tin (beta tin)
- rhombic tin (gamma)
- ferrite (alpha iron) – forms below 770°C (the Curie point, TC); the iron becomes magnetic in its alpha form; BCC
- beta – forms below 912°C (BCC)
- gamma – forms below 1,394°C; face centred cubic (FCC) crystal structure
- delta – forms from cooling down molten iron below 1,538°C; has a body-centred cubic (BCC) crystal structure
Lanthanides and actinides
- Cerium, samarium, terbium, dysprosium and ytterbium have three allotropes.
- Praseodymium, neodymium, gadolinium and terbium have two allotropes.
- Plutonium has six distinct solid allotropes under “normal” pressures. Their densities vary within a ratio of some 4:3, which vastly complicates all kinds of work with the metal (particularly casting, machining, and storage). A seventh plutonium allotrope exists at very high pressures. The transuranium metals Np, Am, and Cm are also allotropic.
- Promethium, americium, berkelium, californium have 3 allotropes [4]
See also
References
- ^ Allotrope in IUPAC Compendium of Chemical Terminology, Electronic/ version, http://goldbook.iupac.org/A00243.html. Accessed March 2007.
- ^ Jensen, W. B. (2006), “The Origin of the Term Allotrope”, J. Chem. Educ. 83 (6): 838–39, Bibcode 2006JChEd..83..838J, doi:10.1021/ed083p838.
- ^ “allotropy”, A New English Dictionary on Historical Principles, 1, Oxford University Press, 1888, p. 238.
- ^ http://www.iop.org/EJ/article/0305-4608/15/2/002/jfv15i2pL29.pdf?request-id=AFlRqDDL3BGhbarg2wi7Kg
![]() Chisholm, Hugh, ed (1911). “Allotropy“. Encyclopædia Britannica (11th ed.). Cambridge University Press.
Chisholm, Hugh, ed (1911). “Allotropy“. Encyclopædia Britannica (11th ed.). Cambridge University Press.
External links
This information originally retrieved from http://en.wikipedia.org/wiki/Allotropes
on Monday 17th October 2011 10:02 am EDT
Now edited and maintained by ManufacturingET.org