Important Link: Periodic Table (Learner.org)
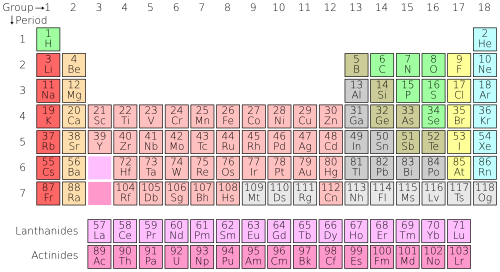
The periodic table (or, more formally, the periodic table of the elements or periodic table of the chemical elements) is a tabular display organizing the 118 known chemical elements by selected properties of their atomic structure. Elements are presented in the periodic table by increasing values of their atomic numbers, the number of protons in their atomic nuclei. While rectangular in general outline, counter-intuitive gaps are included in the horizontal rows (“periods”) as needed to keep elements with similar properties together in each vertical column (“group”), such as the alkali metals, the alkali earths, the halogens, and the noble gases.[1]
The following presentation of the periodic table includes links to Wikipedia articles on each of the 118 included elements:
Group # |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Period |
||||||||||||||||||
1 |
1H |
2He |
||||||||||||||||
2 |
3Li |
4Be |
5B |
6C |
7N |
8O |
9F |
10Ne |
||||||||||
3 |
11Na |
12Mg |
13Al |
14Si |
15P |
16S |
17Cl |
18Ar |
||||||||||
4 |
19K |
20Ca |
21Sc |
22Ti |
23V |
24Cr |
25Mn |
26Fe |
27Co |
28Ni |
29Cu |
30Zn |
31Ga |
32Ge |
33As |
34Se |
35Br |
36Kr |
5 |
37Rb |
38Sr |
39Y |
40Zr |
41Nb |
42Mo |
43Tc |
44Ru |
45Rh |
46Pd |
47Ag |
48Cd |
49In |
50Sn |
51Sb |
52Te |
53I |
54Xe |
6 |
55Cs |
56Ba |
* |
72Hf |
73Ta |
74W |
75Re |
76Os |
77Ir |
78Pt |
79Au |
80Hg |
81Tl |
82Pb |
83Bi |
84Po |
85At |
86Rn |
7 |
87Fr |
88Ra |
** |
104Rf |
105Db |
106Sg |
107Bh |
108Hs |
109Mt |
110Ds |
111Rg |
112Cn |
113Uut |
114Uuq |
115Uup |
116Uuh |
117Uus |
118Uuo |
* Lanthanides (Lanthanoids) |
57La |
58Ce |
59Pr |
60Nd |
61Pm |
62Sm |
63Eu |
64Gd |
65Tb |
66Dy |
67Ho |
68Er |
69Tm |
70Yb |
71Lu |
|||
** Actinides (Actinoids) |
89Ac |
90Th |
91Pa |
92U |
93Np |
94Pu |
95Am |
96Cm |
97Bk |
98Cf |
99Es |
100Fm |
101Md |
102No |
103Lr |
|||
This common arrangement of the periodic table separates the lanthanides (lanthanoids) and actinides (actinoids) (the f-block) from other elements. The wide periodic table incorporates the f-block. The extended periodic table adds the 8th and 9th periods, incorporating the f-block and adding the theoretical g-block.
|
Element categories in the periodic table
|
|||||||||||||||||||||||
|
|
||||||||||||||||||||||
Although there were precursors to this tabular presentation, its invention is generally credited to Russian chemist Dmitri Mendeleev, who developed a version of the now-familiar tabular presentation in 1869 to illustrate recurring (“periodic”) trends in the properties of the then-known elements.[2] The layout of the table has been refined and extended over time, as new elements have been discovered, and new theoretical models have been developed to explain chemical behavior.[3]
Since the periodic table powerfully predicts the abilities of various elements to combine into chemical compounds, use of the periodic table is now ubiquitous within the academic discipline of chemistry, providing a useful framework to classify, systematize, and compare all of the many different forms of chemical behavior. The table has found many applications not only in chemistry and physics, but also in such diverse fields as geology, biology, materials science, engineering, agriculture, medicine, nutrition, environmental health, and astronomy. Its principles are especially important in chemical engineering.
One of the strengths of Mendeleev’s original presentation was the prediction of the properties of then-undiscovered elements expected to fill noticeable gaps in the arrangement, such as his “eka-aluminium” with properties intermediate between aluminium and indium, which was discovered in 1875 and named gallium. No gaps remain in the current 118-element periodic table, since all of the 91 regularly occurring chemical elements[4] have now been isolated, characterized, and named, and technetium, promethium, and all of the transuranic elements through 118 have been synthesized.
While plutonium is now included among the regularly occurring natural elements, and technetium, promethium, and neptunium also occur naturally, but only incidentally, these four elements were first identified and characterized from technologically produced samples. Numerous synthetic radionuclides of various naturally occurring elements have also been produced. Production of additional synthetic elements beyond atomic number 118 is being pursued; their properties are expected to continue the periodic patterns shown by the presently known elements.
Contents |
Organizing principles
The main value of the periodic table is the ability to predict the chemical properties of an element based on its location on the table. It should be noted that the properties vary differently when moving vertically along the columns of the table than when moving horizontally along the rows.[1]
The layout of the periodic table demonstrates recurring (“periodic”) chemical properties. Elements are listed in order of increasing atomic number (i.e., the number of protons in the atomic nucleus). Rows are arranged so that elements with similar properties fall into the same columns (groups or families). According to quantum mechanical theories of electron configuration within atoms, each row (period) in the table corresponded to the filling of a quantum shell of electrons. There are progressively longer periods further down the table, grouping the elements into s-, p-, d- and f-blocks to reflect their electron configuration.[1]
Elements, natural and synthetic
Only chemical elements, not mixtures, compounds, or subatomic particles, are included in the periodic table. Each element has a single entry, even if it has multiple isotopes.[1]
As of June 2011, the periodic table includes 118 chemical elements whose discoveries have been confirmed. Of these, 91 are regularly occurring primordial or recurrently produced elements found naturally on the Earth, at least in transient trace amounts, and three others occur naturally, but only incidentally.[1][4] The 24 other known elements (those from americium through ununoctium) are synthetic, produced by human technology but not regularly or incidentally occurring naturally.[1] Various synthetic elements, as well as synthetic isotopes of naturally occurring elements, are now also present in the environment from such sources as nuclear weapons explosions, nuclear waste processing, and disposal of materials including industrial and medical nucleotides. For example, americium and its decay product neptunium are incidentally present in household and commercial waste from disposal of unwanted americium-containing smoke detectors.
Formal naming of the chemical elements is overseen by the International Union of Pure and Applied Chemistry (IUPAC). Provisional names, such as ununtrium, ununquadium, or ununpentium, are provided for elements that have been discovered but not yet been formally named; these names are based on the three digits of their atomic numbers.[1][5]
Atomic number
By definition, each chemical element has a unique atomic number, the number of protons in its nucleus. Different atoms of many elements have different numbers of neutrons, which differentiates between isotopes of an element. For example, all atoms of hydrogen have one proton, and no atoms of any other element have exactly one proton. On the other hand, a hydrogen atom can have one or two neutrons in its nucleus, or none at all, yet all of these cases are isotopes of hydrogen, not instances of some other element. (A hydrogen atom with no neutrons in addition to its sole proton is called protium, one with one neutron in addition to its proton is called deuterium, and one with two additional neutrons, tritium.)
In the modern periodic table, the elements are placed progressively in each row (period) from left to right in the sequence of their atomic numbers, with each new row starting with the next atomic number following the last number in the previous row. No gaps or duplications exist. Since the elements can be uniquely sequenced by atomic number, conventionally from lowest to hightest, sets of elements are sometimes specified by such notation as “through”, “beyond”, or “from … through”, as in “through iron”, “beyond uranium”, or “from lanthanum through lutetium”. The terms “light” and “heavy” are sometimes also used informally to indicate relative atomic numbers (not densities!), as in “lighter than carbon” or “heavier than lead”, although technically the weight or mass of atoms of an element (their atomic weights or atomic masses) do not always increase monotonically with their atomic numbers.
The significance of atomic numbers to the organization of the periodic table was not appreciated until the existence and properties of protons and neutrons became understood. Mendeleev’s periodic tables instead used atomic weights, information determinable to fair precision in his time, which worked well enough in most cases to give a powerfully predictive presentation far better than any other comprehensive portrayal of the chemical elements’ properties then possible. Substitution of atomic numbers, once understood, gave a definitive, integer-based sequence for the elements, still used today even as new synthetic elements are being produced and studied.
Periodicity of chemical properties
The primary determinant of an element’s chemical properties is its electron configuration, particularly the valence shell electrons. For instance, any atoms with four valence electrons occupying p orbitals will exhibit some similarity. The type of orbital in which the atom’s outermost electrons reside determines the “block” to which it belongs. The number of valence shell electrons determines the family, or group, to which the element belongs.[1]
| Subshell | S | G | F | D | P |
|---|---|---|---|---|---|
| Period | |||||
| 1 | 1s | ||||
| 2 | 2s | 2p | |||
| 3 | 3s | 3p | |||
| 4 | 4s | 3d | 4p | ||
| 5 | 5s | 4d | 5p | ||
| 6 | 6s | 4f | 5d | 6p | |
| 7 | 7s | 5f | 6d | 7p | |
| 8 | 8s | 5g | 6f | 7d | 8p |
The total number of electron shells an atom has determines the period to which it belongs. Each shell is divided into different subshells, which as atomic number increases are filled in roughly this order (the Aufbau principle) (see table).[6] Hence the structure of the periodic table. Since the outermost electrons determine chemical properties, those with the same number of valence electrons are generally grouped together.[1]
Progressing through a group from lightest element to heaviest element, the outer-shell electrons (those most readily accessible for participation in chemical reactions) are all in the same type of orbital, with a similar shape, but with increasingly higher energy and average distance from the nucleus. For instance, the outer-shell (or “valence“) electrons of the first group, headed by hydrogen, all have one electron in an s orbital. In hydrogen, that s orbital is in the lowest possible energy state of any atom, the first-shell orbital (and represented by hydrogen’s position in the first period of the table).[7] In francium, the heaviest element of the group, the outer-shell electron is in the seventh-shell orbital, significantly further out on average from the nucleus than those electrons filling all the shells below it in energy. As another example, both carbon and lead have four electrons in their outer shell orbitals.[1]
Note that as atomic number (i.e., charge on the atomic nucleus) increases, this leads to greater spin-orbit coupling between the nucleus and the electrons, reducing the validity of the quantum mechanical orbital approximation model, which considers each atomic orbital as a separate entity.[citation needed]
Groups
A group or family is a vertical column in the periodic table. Groups are considered the most important method of classifying the elements. In some groups, the elements have very similar properties and exhibit a clear trend in properties down the group. Under the international naming system, the groups are numbered numerically 1 through 18 from the left most column (the alkali metals) to the right most column (the noble gases).[8] The older naming systems differed slightly between Europe and America (the table shown in this section shows the old American Naming System).[9]
Some of these groups have been given trivial (unsystematic) names, such as the alkali metals, alkaline earth metals, halogens, pnictogens, chalcogens, and noble gases. However, some other groups, such as group 7, have no trivial names and are referred to simply by their group numbers, since they display fewer similarities and/or vertical trends.[8]
Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group generally have the same electron configurations in their valence shell, which is the most important factor in accounting for their similar properties.[1]
Elements in the same group show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, valence electrons are found farther from the nucleus. From the top, each successive element has a lower ionization energy because it is easier to remove an electron since the atoms are less tightly bound. Similarly, a group has a top to bottom decrease in electronegativity due to an increasing distance between valence electrons and the nucleus.[10]
Periods
A period is a horizontal row in the periodic table. Although groups are the most common way of classifying elements, there are some regions of the periodic table where the horizontal trends and similarities in properties are more significant than vertical group trends. This can be true in the d-block (or “transition metals“), and especially for the f-block, where the lanthanides and actinides form two substantial horizontal series of elements.[citation needed]
Periodic trend for ionization energy. Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases.
Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus.[11] This decrease in atomic radius also causes the ionization energy to increase when moving from left to right across a period. The more tightly bound an element is, the more energy is required to remove an electron. Electronegativity increases in the same manner as ionization energy because of the pull exerted on the electrons by the nucleus.[10] Electron affinity also shows a slight trend across a period. Metals (left side of a period) generally have a lower electron affinity than nonmetals (right side of a period) with the exception of the noble gases.[12]
Blocks
This diagram shows the periodic table blocks with the CAS (American Group Numbering System).
Because of the importance of the outermost electron shell, the different regions of the periodic table are sometimes referred to as periodic table blocks, named according to the subshell in which the “last” electron resides. The s-block comprises the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p-block comprises the last six groups which are groups 13 through 18 in IUPAC (3A through 8A in American) and contains, among others, all of the semimetals. The d-block comprises groups 3 through 12 in IUPAC (or 3A through 8A in American group numbering) and contains all of the transition metals. The f-block, usually offset below the rest of the periodic table, comprises the lanthanides and actinides.[13]
Conventional and alternative formats
The periodic table as commonly presented, with horizontal periods, vertical groups, and highlighting to show similar elements. Rather than being incorporated in their proper places, the lanthanides and actinides are here shown in separate rows beneath the other elements, providing a more convenient (and aesthetically more pleasing), but less accurate, layout.
In printed or other formally presented periodic tables, each element is provided a formatted cell that provides selected information on each element. Atomic number, element symbol, and name, are generally included, as well as selected other information, such as each element’s atomic weight, density, melting and boiling points, crystal structure as a solid, origin, abbreviated electron configuration, electronegativity, and most common valence numbers.[14]
The information included in a periodic table can be presented in many ways, including selection of kinds of data to be shown, layout within the cells representing particular elements, and the format used to present the table’s periodic patterns. Colors, symbols, and other formatting conventions are often used in periodic tables to show selected additional information for each element compactly. Interactive versions may also include hyperlinks to additional information, as in the version shown at the top of this Wikipedia article.
Sculpture of the periodic table in circular layout, with the portrait of Dmitri Mendeleev in the middle (Bratislava, Slovakia). The table is shown to be almost circular even though most commonly it is not drawn so.
While the iconic format presented above is widely used,[1] other alternative periodic tables exist, including not only various rectangular formats, but also circular or cylindrical versions in which the rows (periods) flow from one into another, without the arbitrary breaks required at the margins of the usual printed or screen-formatted versions.
In presentations of the periodic table, the lanthanides and the actinides are customarily shown as two additional rows below the main body of the table,[1] with placeholders or else a selected single element of each series (either lanthanum or lutetium, and either actinium or lawrencium, respectively) shown in a single cell of the main table, between barium and hafnium, and radium and rutherfordium, respectively. This convention is entirely a matter of aesthetics and formatting practicality; a rarely used wide-formatted periodic table inserts the lanthanide and actinide series in their proper places, as parts of the table’s sixth and seventh rows (periods).
Many presentations of the periodic table show a dark stair-step diagonal line along the metalloids, with metals to the left of the line and non-metals to the right.[1][15] Various other groupings of the chemical elements are sometimes also highlighted on a periodic table, such as transition metals, poor metals, and metalloids. Other informal groupings of the elements exist, such as the platinum group and the noble metals, but are rarely addressed in periodic tables.[citation needed]
Hydrogen is usually placed above lithium, although its chemistry differs substantially from that of lithium and the other alkali metals; some periodic tables place it on its own.[1]
Elements with atomic numbers greater than 82, as well as technetium and promethium, have no stable isotopes; the atomic mass of each of these element’s isotope having the longest half-life is typically reported on periodic tables with parentheses.[16]
History
Mendeleev‘s 1869 periodic table; note that his arrangement presents the periods vertically, and the groups horizontally
In 1789, Antoine Lavoisier published a list of 33 chemical elements. Although Lavoisier grouped the elements into gases, metals, non-metals, and earths, chemists spent the following century searching for a more precise classification scheme. In 1829, Johann Wolfgang Döbereiner observed that many of the elements could be grouped into triads (groups of three) based on their chemical properties. Lithium, sodium, and potassium, for example, were grouped together as being soft, reactive metals. Döbereiner also observed that, when arranged by atomic weight, the second member of each triad was roughly the average of the first and the third.[17] This became known as the Law of Triads.[18] German chemist Leopold Gmelin worked with this system, and by 1843 he had identified ten triads, three groups of four, and one group of five. Jean Baptiste Dumas published work in 1857 describing relationships between various groups of metals. Although various chemists were able to identify relationships between small groups of elements, they had yet to build one scheme that encompassed them all.[17]
German chemist August Kekulé had observed in 1858 that carbon has a tendency to bond with other elements in a ratio of one to four. Methane, for example, has one carbon atom and four hydrogen atoms. This concept eventually became known as valency. In 1864, fellow German chemist Julius Lothar Meyer published a table of the 49 known elements arranged by valency. The table revealed that elements with similar properties often shared the same valency.[19]
English chemist John Newlands produced a series of papers in 1864 and 1865 that described his own classification of the elements: he noted that when listed in order of increasing atomic weight, similar physical and chemical properties recurred at intervals of eight, which he likened to the octaves of music.[20][21] This Law of Octaves, however, was ridiculed by his contemporaries, and the Chemical Society refused to publish his work.[22] Nonetheless, Newlands was able to draft an atomic table and use it to predict the existence of missing elements, such as germanium. The Chemical Society only acknowledged the significance of his discoveries some five years after they credited Mendeleev.
Russian chemistry professor Dmitri Ivanovich Mendeleev and German chemist Julius Lothar Meyer independently published their periodic tables in 1869 and 1870, respectively. They both constructed their tables in a similar manner: by listing the elements in a row or column in order of atomic weight and starting a new row or column when the characteristics of the elements began to repeat.[23] The success of Mendeleev’s table came from two decisions he made: The first was to leave gaps in the table when it seemed that the corresponding element had not yet been discovered.[24] Mendeleev was not the first chemist to do so, but he was the first to be recognized as using the trends in his periodic table to predict the properties of those missing elements, such as gallium and germanium.[25] The second decision was to occasionally ignore the order suggested by the atomic weights and switch adjacent elements, such as cobalt and nickel, to better classify them into chemical families. With the development of theories of atomic structure, it became apparent that Mendeleev had listed the elements in order of increasing atomic number.[26]
With the development of modern quantum mechanical theories of electron configurations within atoms, it became apparent that each row (or period) in the table corresponded to the filling of a quantum shell of electrons. In Mendeleev’s original table, each period was the same length. However, because larger atoms have more electron sub-shells, modern tables have progressively longer periods further down the table.[27]
In the years following publication of Mendeleev’s periodic table, the gaps he identified were filled as chemists discovered additional naturally occurring elements. It is often stated that the last naturally occurring element to be discovered was francium (referred to by Mendeleev as eka-caesium) in 1939.[28] However, plutonium, produced synthetically in 1940, was identified in trace quantities as a naturally occurring primordial element in 1971.[29]
The production of various transuranic elements has expanded the periodic table significantly, the first of these being neptunium, synthesized in 1939.[30] Because many of the transuranic elements are highly unstable and decay quickly, they are challenging to detect and characterize when produced, and there have been controversies concerning the acceptance of competing discovery claims for some elements, requiring independent review to determine which party has priority, and hence naming rights. The most recently named element is copernicium (number 112), named on 19 February 2010;[31] the most recently accepted discoveries are ununquadium (114) and ununhexium (116), both accepted on 1 June 2011.[32]
See also
| Book: Periodic table | |
| Wikipedia Books are collections of articles that can be downloaded or ordered in print. | |
- Alternative periodic tables
- List of Periodic table related articles
- Abundance of the chemical elements
- Atomic electron configuration table
- Discoveries of the chemical elements
- Electronegativity
- Element collecting
- Extended periodic table
- History of the periodic table
- IUPAC‘s systematic element names
- Periodic group
- Chemical elements in East Asian languages
- Table of chemical elements
- Table of nuclides
- Periodic Matrix Sets
- Photovoltaic effect
References
- ^ a b c d e f g h i j k l m n o Gray, Theodore (2009). The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal Publishers. pp. 240. ISBN 978-1-57912-814-2.
- ^ Dimitri Mendelejew: Ueber die Beziehungen der Eigenschaften zu den Atomgewichten der Elemente. In: Zeitschrift für Chemie. 1869, pp. 405–406.
- ^ IUPAC article on periodic table
- ^ a b This definition of regularly occurring natural elements includes all elements from hydrogen to plutonium except technetium, promethium and neptunium, all of which always exist in the Earth in macroscopic or recurrently produced trace quantities. The three exceptions do exist naturally, but only incidentally, produced as the result of rare nuclear processes from heavy elements.
- ^ Koppenol, W. H. (2002). “Naming of New Elements (IUPAC Recommendations 2002)” (PDF). Pure and Applied Chemistry 74 (5): 787–791. http://media.iupac.org/publications/pac/2002/pdf/7405×0787.pdf.
- ^ Moore, p. 46
- ^ Hornback, Joseph (2006). Organic Chemistry (2nd ed.). Pacific Grove: Thomson Brooks/Cole. p. 62. ISBN 978-0-534-49317-2. OCLC 66441248.
- ^ a b Leigh, G. J. Nomenclature of Inorganic Chemistry: Recommendations 1990. Blackwell Science, 1990. ISBN 0632024941.
- ^ Leigh, Jeffery. “Periodic Tables and IUPAC”. Chemistry International: The News Magazine of The International Union of Pure and Applied Chemistry (IUPAC). http://www.iupac.org/publications/ci/2009/3101/1_leigh.html. Retrieved 23 March 2011.
- ^ a b Moore, p. 111
- ^ Mascetta, Joseph (2003). Chemistry The Easy Way (4th ed.). New York: Hauppauge. p. 50. ISBN 9780764119781. OCLC 52047235.
- ^ Kotz, John; Treichel, Paul; Townsend, John (2009). Chemistry and Chemical Reactivity, Volume 2 (7th ed.). Belmont: Thomson Brooks/Cole. p. 324. ISBN 978-0-495-37812-1. OCLC 220756597.
- ^ Jones, Chris (2002). d- and f-block chemistry. New York: J. Wiley & Sons. p. 2. ISBN 9780471224761. OCLC 300468713.
- ^ An example (among many) showing several of these descriptors: (Plasticized placemat) Painless Learning Placemats: Periodic Table of the Elements. M. Ruskin Co.. 2000. pp. 2.
- ^ Science Standards of Learning Curriculum Framework
- ^ Dynamic periodic table
- ^ a b Ball, p. 100
- ^ Horvitz, Leslie (2002). Eureka!: Scientific Breakthroughs That Changed The World. New York: John Wiley. p. 43. ISBN 9780471233411. OCLC 50766822.
- ^ Ball, p. 101
- ^ Newlands, John A. R. (1864-08-20). “On Relations Among the Equivalents”. Chemical News 10: 94–95. http://web.lemoyne.edu/~giunta/EA/NEWLANDSann.HTML#newlands3.
- ^ Newlands, John A. R. (1865-08-18). “On the Law of Octaves”. Chemical News 12: 83. http://web.lemoyne.edu/~giunta/EA/NEWLANDSann.HTML#newlands4.
- ^ Bryson, Bill (2004). A Short History of Nearly Everything. London: Black Swan. pp. 141–142. ISBN 9780552151740.
- ^ Ball, pp. 100–102
- ^ Pullman, Bernard (1998). The Atom in the History of Human Thought. Translated by Axel Reisinger. Oxford University Press. p. 227. ISBN 0-19-515040-6.
- ^ Ball, p. 105
- ^ Atkins, P. W. (1995). The Periodic Kingdom. HarperCollins Publishers, Inc.. p. 87. ISBN 0-465-07265-8.
- ^ Ball, p. 111
- ^ Adloff, Jean-Pierre; Kaufman, George B. (2005-09-25). Francium (Atomic Number 87), the Last Discovered Natural Element. The Chemical Educator 10 (5). Retrieved on 2007-03-26.
- ^ Hoffman, D. C.; Lawrence, F. O.; Mewherter, J. L.; Rourke, F. M. (1971). “Detection of Plutonium-244 in Nature”. Nature 234 (5325): 132–134. Bibcode 1971Natur.234..132H. doi:10.1038/234132a0. http://www.nature.com/nature/journal/v234/n5325/abs/234132a0.html.
- ^ Ball, p. 123
- ^ “[IUPAC]Element 112 is Named Copernicium”. iupac.org. doi:10.1351/PAC-REP-08-03-05. http://www.iupac.org/web/nt/2010-02-20_112_Copernicium. Retrieved 2010-06-12.
- ^ Barber, Robert C.; Karol, Paul J; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich W. (2011). “Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)”. Pure Appl. Chem.. doi:10.1351/PAC-REP-10-05-01.
Bibliography
- Ball, Philip (2002). The Ingredients: A Guided Tour of the Elements. Oxford University Press. ISBN 0-19-284100-9.
- Moore, John (2003). Chemistry For Dummies. New York: Wiley Publications. p. 111. ISBN 978-0-7645-5430-8. OCLC 51168057.
Further reading
- Bouma, J. (1989). “An Application-Oriented Periodic Table of the Elements”. J. Chem. Ed. 66: 741. Bibcode 1989JChEd..66..741B. doi:10.1021/ed066p741.
- Hjørland, Birger (2011). “The periodic table and the philosophy of classification”. Knowledge Organization 38 (1): 9–21. http://ucla.academia.edu/EricScerri/Papers/432740/Forum%5FThe%5FPhilosophy%5Fof%5FClassification. Retrieved 2011-03-13.
- Mazurs, E.G (1974). Graphical Representations of the Periodic System During One Hundred Years. Alabama: University of Alabama Press.
- Scerri, Eric (2007). The periodic table: its story and its significance. Oxford: Oxford University Press. ISBN 0-19-530573-6.
External links
| Find more about Periodic table on Wikipedia’s sister projects: | |
| Definitions from Wiktionary | |
| Images and media from Commons | |
| Learning resources from Wikiversity | |
| News stories from Wikinews | |
| Quotations from Wikiquote | |
| Source texts from Wikisource | |
| Textbooks from Wikibooks | |
- Interactive periodic table
- Video periodic table
- WebElements
- IUPAC periodic table
- 118 elements: The Periodic Table of Videos made by Brady Haran, featuring Martyn Poliakoff and others, at the University of Nottingham.
- A catalog of various forms of the periodic table
| Periodic table | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | |||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | |||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||
This information originally retrieved from http://en.wikipedia.org/wiki/Periodic_table
on Wednesday 3rd August 2011 11:45 am EDT
Now edited and maintained by ManufacturingET.org